Cancer Gene Therapy Market Size to hit $19.97 Billion by 2033 | Key Drivers, Trends, Precision Oncology & FDA
Cancer Gene Therapy Market Insights | Size, Share & Forecast 2025–2033 | Key Biotech Advances
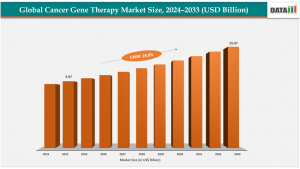
Cancer Gene Therapy Market in USA Hits $3.97B in 2024, Projected to Surpass $19.97B by 2033”
AUSTIN, TX, UNITED STATES, October 30, 2025 /EINPresswire.com/ -- Cancer Gene Therapy Market Overview— DataM Intelligence 4Market Research LLP
According to DataM Intelligence Comprehensive report, cancer gene therapy market size grew from US$3.36 billion to US$3.97 billion in 2024 and is projected to reach US$19.97 billion by 2033, registering a CAGR of 19.8% from 2025 to 2033. The growth is underpinned by an increasing incidence of cancer globally, rising demand for targeted therapeutics, and greater investment in gene-based research and clinical trials.
Cancer gene therapy involves the direct transfer of genetic material into a patient’s cells to combat or prevent disease. Instead of merely managing symptoms, these therapies target the root cause by modifying the genetic code within cancer cells or immune cells. The approach includes gene silencing, oncolytic virotherapy, gene augmentation, and CAR-T cell therapy, which have shown significant promise in improving patient outcomes.
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):– https://www.datamintelligence.com/download-sample/cancer-gene-therapy-market
Key Highlights
North America led the global cancer gene therapy market, accounting for the largest revenue share of 43.5% in 2024.
Asia Pacific emerged as the fastest-growing region, projected to expand at a CAGR of 8.1% during the forecast period.
By therapy type, the gene-induced immunotherapy segment dominated the market with a 44.1% revenue share in 2024.
Key Industry Developments
In May 2025, the U.S. FDA approved nine cell and gene therapy products in 2024, targeting conditions such as cancer, hemophilia B, and L-amino acid decarboxylase deficiency. This progress continues into 2025 with approvals for Encelto (macular telangiectasia Type 2) and Zevaskyn (recessive dystrophic epidermolysis bullosa), offering new hope for patients with few treatment options.
In June 2024, the FDA approved a combination therapy of adagrasib and cetuximab for KRAS G12C-mutated colorectal cancer, marking a major milestone in precision oncology.
Meanwhile, in April 2024, India launched its first indigenous CAR T-cell therapy, a revolutionary treatment that reprograms immune cells to target cancer more effectively celebrated by the President of India as a symbol of “new hope” in cancer care.
Market Drivers
1. Increasing Cancer Prevalence
According to the World Health Organization (WHO), cancer accounted for over 10 million deaths in 2023, making it one of the leading global health challenges. With the rise in cancer incidence particularly lung, breast, prostate, and colorectal cancers demand for next-generation therapies has intensified. Gene therapy offers a more durable and potentially curative solution compared to conventional chemotherapy or radiation.
2. Technological Breakthroughs in Gene Editing
The evolution of gene editing platforms such as CRISPR-Cas9, TALEN, and zinc finger nucleases has transformed the precision and efficiency of gene modification. These tools allow researchers to edit disease-causing genes with remarkable accuracy, reducing off-target effects and accelerating clinical translation. For instance, CRISPR-based cancer immunotherapies are now being tested in multiple late-stage trials for solid tumors and hematologic malignancies.
3. Growth in Clinical Approvals and Research Investments
The FDA and EMA have increased approvals for gene-based therapies targeting rare and oncology indications. In 2024, the U.S. FDA approved multiple CAR-T and oncolytic virus therapies for hematologic cancers, demonstrating the maturing regulatory landscape. Pharmaceutical giants such as Novartis, Gilead Sciences, and Bristol Myers Squibb continue to expand their oncology pipelines through mergers, licensing, and R&D investments in genetic medicine.
Regional Analysis
North America (43.5% share, 2024)
North America leads the global cancer gene therapy market, driven by high healthcare spending, strong clinical research infrastructure, and rapid adoption of advanced oncology treatments. The U.S. dominates with FDA-approved breakthroughs like Kymriah, Yescarta, and Tecelra, backed by robust funding, manufacturing capabilities, and active clinical trials.
Europe (34.5% share, 2024)
Europe holds the second-largest market share, supported by strong R&D, collaborative innovation, and supportive regulations. The EIC Cell & Gene Therapy Symposium (2023) accelerated regional growth, with Germany and the UK emerging as key hubs due to cutting-edge research, clinical activity, and rising cancer incidence.
Asia Pacific (CAGR 8.1%)
Asia Pacific is the fastest-growing region, propelled by improving healthcare systems, rising cancer cases, and pro-biotech policies. Japan leads with early approvals like Tecartus, fast-track regulations, and strong government–industry collaboration—positioning it as a global frontrunner in cancer gene therapy innovation.
Key Players
1. Prominent players include
2. Novartis AG
3. Pfizer
4. Adapt immune Therapeutics
5. Bluebird Bio, Inc.
6. Krystal Biotech, Inc.
7. Regulus Therapeutics
8. Biogenera
M&A activity continues to shape the market. For instance, in 2025, Gilead Sciences announced the acquisition of a gene-editing startup to enhance its precision oncology portfolio, highlighting the sector’s consolidation trend.
Get Customization in the report as per your requirements:- https://www.datamintelligence.com/customize/cancer-gene-therapy-market
Market Segmentation
By Technique: (In vivo Gene Therapy, Ex vivo Gene Therapy)
By Therapy: (Gene Induced Immunotherapy, Oncolytic Virotherapy, Gene Transfer, Others)
By Application: (Brain Cancer, Lung Cancer, Breast Cancer, Pancreatic Cancer, Liver Cancer, Others)
By End-User: (Hospitals, Cancer Centers, Others)
By Region: (North America, Europe, South America, Asia Pacific, Middle East, and Africa)
Have any Query? Talk to our Expert @ https://www.datamintelligence.com/enquiry/cancer-gene-therapy-market
Challenges and Restraints
Despite remarkable progress, cancer gene therapy faces notable challenges:
High development and manufacturing costs, particularly in vector production and patient-specific cell modification.
Complex regulatory pathways across different regions for approval and post-market surveillance.
Safety and ethical considerations around gene editing, including potential off-target mutations and immune responses.
Limited accessibility and reimbursement, as many gene therapies remain prohibitively expensive for widespread clinical use.
Addressing these barriers requires cross-sector collaboration, innovative pricing models, and advances in scalable manufacturing technologies.
DataM Intelligence Insights and Recommendations
DataM Intelligence identifies several key strategies for stakeholders to maximize market potential:
Invest in scalable vector platforms – Companies should prioritize modular viral and non-viral delivery systems to cut production costs and accelerate clinical rollout.
Strengthen strategic partnerships – Collaborations between biotech firms, academic institutions, and contract development and manufacturing organizations (CDMOs) will speed innovation and regulatory compliance.
Focus on personalization – Leveraging AI-driven genomic analytics and biomarker profiling can enhance therapy precision and patient outcomes.
Expand access through health-economic models – Introducing outcome-based payment schemes and regional manufacturing hubs will improve affordability.
Enhance regulatory alignment – Early engagement with agencies like the FDA, EMA, and PMDA can streamline approval timelines.
Conclusion
The Cancer Gene Therapy Market stands at a pivotal inflection point, combining the power of genetics, data science, and biotechnology to redefine oncology treatment. While challenges remain, the long-term outlook is exceptionally positive. With increasing clinical successes and investor confidence, gene therapy is poised to transition from an experimental approach to a mainstream cancer treatment strategy by 2032.
DataM Intelligence forecasts sustained market growth, driven by scientific innovation, evolving reimbursement frameworks, and the ongoing convergence of genomics and precision oncology
Buy Now & Unlock 360° Market Intelligence:- https://www.datamintelligence.com/buy-now-page?report=cancer-gene-therapy-market
Related Reports
Cell Therapy Market
Cell and Gene Therapy Market
Sai Kiran
DataM Intelligence 4Market Research LLP
+1 877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.